

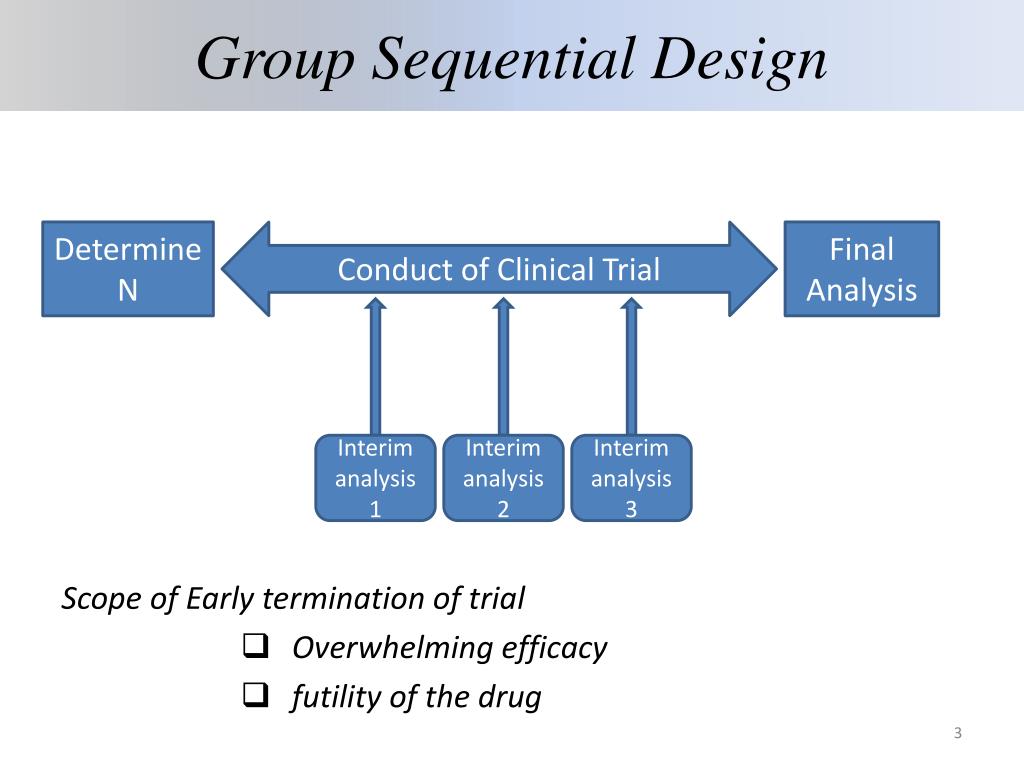
If the test statistic does not meet the threshold, the trial continues. 3 During the interim analysis, a test statistic is calculated (eg, a P value), and, if the test statistic meets a prespecified threshold, the trial may stop. One action that may be triggered by an interim analysis is stopping enrollment due to clear evidence that the investigational treatment is superior (known as early success). This generally includes when the interim analyses will occur, what information will be used, and what statistical methods and stopping criteria will be applied. When planning a group sequential trial, investigators must prespecify plans for interim analyses. This allows the trial to be stopped and a valid conclusion to be drawn earlier.ĭescription of Interim Analyses in Group Sequential Trials

The data available at the time of the interim analysis are assessed to determine if predefined stopping criteria are met. Group sequential trials incorporate interim analyses to allow timely decisions that mitigate the challenges associated with uncertainty in the size and direction of the treatment effect.
#GROUP SEQUENTIAL TESTING FULL#
In addition, because new treatments may be ineffective or even harmful relative to standard care, enrolling a trial to its full sample size without reviewing the data risks could waste resources or even harm patients. Alternatively, if the true treatment effect is overestimated, the calculated sample size may be too small, resulting in an underpowered trial that may fail to detect a real benefit. 2 If the true treatment effect is underestimated, the calculated sample size may be larger than necessary, increasing the time needed to complete the trial and potentially delaying delivery of effective treatments to patients outside the study. The assumed treatment effect (the magnitude of difference the trial is designed to reliably detect) strongly affects the number of patients needed for the trial. There is substantial uncertainty regarding the likely treatment effect (ie, the between-group difference in outcomes) when a clinical trial is being designed. Why Are Interim Analyses Performed in Group Sequential Trials? Shared Decision Making and Communication.Scientific Discovery and the Future of Medicine.Health Care Economics, Insurance, Payment.Clinical Implications of Basic Neuroscience.Challenges in Clinical Electrocardiography.


 0 kommentar(er)
0 kommentar(er)
